Synovex® Plus
Reg. No.: G2379 (Act 36/1947)
For animal use only.

Home / Products / Beef and Feedlot / Synovex® Plus

Implant.
200 mg Trenbolone Acetate and 28 mg Oestradiol Benzoate per implant.
Contains trenbolone acetate and oestradiol benzoate which together increase average daily gain, improve feed efficiency and promote the production of lean meat in steers fed in confinement for slaughter.
There is no withdrawal period.
Keep out of reach of children, uninformed persons and pets.
Do not use in animals intended for subsequent breeding or in dairy animals.
Only for use in cattle in feedlot or fattening pens.
Handle with care. Harmful if swallowed.
Implant pellets in the ear only; any other location may result in condemnation of the carcass. Do not attempt salvage of implanted site for human or animal food.
Although this remedy has been extensively tested under a large variety of conditions, failure thereof may ensue as a result of a wide range of reasons. If this is suspected, seek veterinary advice and notify the registration holder.
Store away from food and feed.
Use only as directed.
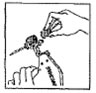
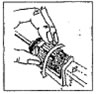
Disinfect the needle before each new implantation by wiping it with gauze saturated with methylated spirits or other approved disinfectant. Never sacrifice careful, clean technique for speed of implantation. Clean the implantation site (back side of the ear) with gauze soaked in disinfectant.
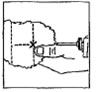
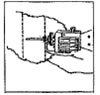
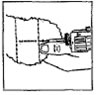
Dosage: One implant (eight pellets) is administered to each animal by subcutaneous implantation in the middle third of the ear with the Synovex® implant applicator. Implant complete contents of one cartridge cell per animal. Maintain pressure for a few seconds on the puncture wound after needle withdrawal.
 |
|
|||||
 |
|
|||||
 |
|
|||||
 |
|
|||||
 |
|
|||||
|
NB: Implant complete contents of one treatment unit (8 pellets) per animal. |
||||||
 |
|
|||||
Keep all parts of instrument lightly oiled when not in use.
It is recommended that the needle of the applicator be disinfected after each implant. This procedure, when properly executed, will minimise the possible transmission of disease from animal to animal. Follow the manufactu
Store unopened product in a cool, dry place. Avoid excessive heat and humidity. Use product before the expiration date printed on foil pouch. Once the pouch is opened, unused product may be stored in the ziplock pouch (away from light) for up to 6 months under refrigerated conditions or between 15 °C - 30 °C for up to 1 month.
Plastic cartridge containing 10 rows of 8 pellets each (10 doses). Ten cartridges are packed per aluminium pouch (100 doses). Pouch is contained within an outer carton with leaflet. The Synovex® applicator has to be obtained separately
DISCLAIMER: ALTHOUGH GREAT CARE HAS BEEN TAKEN IN THE COMPILATION OF THIS DOCUMENT, READERS ARE REQUESTED TO REFER TO THE PACKAGE INSERT FOR COMPLETE DETAILS BEFORE USING THE SPECIFIC PRODUCT.
Ordering Products |
| Place an Order: |
| ZoetisOrders@zoetis.com |
| Find Out About an Order: |
| Zoetis.queries@zoetis.com |
| Find Out About a Delivery: |
| ZoetisDSVqueries@zoetis.com |
| Physical Address: |
| Zoetis South Africa (Pty) Ltd. Co. Reg. No.: 2012/001825/07 |
| 6th Floor, North Wing, 90 Rivonia Road, Sandton, 2196. |
| Tel: |
| 0800 998 969 |
Zoetis South Africa (Pty) Ltd
PostNet Suite 53
Private Bag 9976
Sandton
2146

You are leaving the country website to access another site in the group.
Regulatory constraints and medical practices vary from country to country. Consequently, the information provided on the site in which you enter may not be suitable for use in your country.
