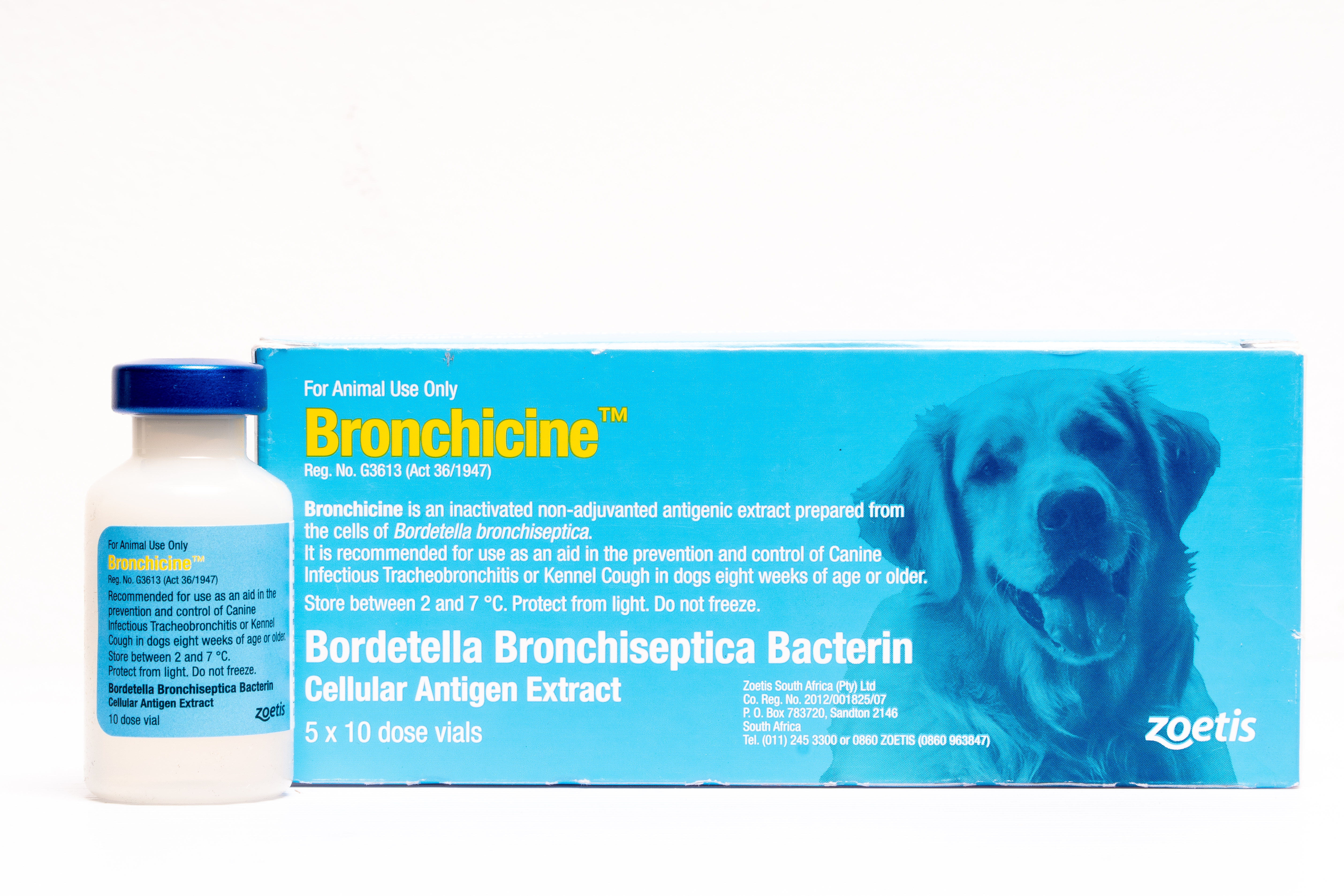
Bronchicine™
For animal use only
Expand All
-
Bronchicine™ is an inactivated, non-adjuvanted antigenic extract, clear, injectable vaccine, prepared from the cells of Bordetella bronchiseptica.
It contains the following:
Thimerosal: This is added to the final concentration of ≤ 1:10,000 to inactivate any residual viable cells and to act as a preservative
Glycerine: 0,1 M final concentration (stabiliser).
Amount of antigen per dose in final container: Each 1 ml dose contains extracted antigen derived from at least 3 x 108 Bordetella bronchiseptica cells.
-
Bronchicine™ is an inactivated non-adjuvanted antigenic extract prepared from the cells of Bordetella bronchiseptica. It is recommended for use as an aid in the prevention and control of Canine Infectious Tracheobronchitis or Kennel Cough in dogs eight weeks of age or older. Although Kennel Cough is considered a disease of complex etiology, it can be reproduced by challenge with Bordetella bronchiseptica alone. A close association and/or confinement of dogs facilitate spread of the disease syndrome. Antibiotic therapy has been shown to be generally unsuccessful in reducing or eliminating Bordetella brochiseptica infection in dogs.
-
For use in dogs only.
Do not vaccinate into blood vessels.
If blood enters the syringe, choose another injection site. Keep out of reach of children and uninformed persons.
Although this vaccine has been extensively tested under a large variety of conditions, failure thereof may ensue as a result of a wide range of reasons. If this is suspected, seek veterinary advice and notify the registration holder.
-
Do not freeze. Use the entire contents when first opened.
Care should be taken to avoid microbial contamination of the product. In case of anaphylactoid reactions, consult a veterinarian.
Transient local reaction at the site of injection, though rare, may occur subsequent to the use of this product.
-
This vaccine should be administered by subcutaneous injection only.
Shake well. Aseptically remove 1 ml from the vial. Push out any air trapped in the syringe. Administer the entire contents (1 ml) subcutaneously under loose skin (back of neck) to healthy dogs, at least 8 weeks of age.
A second dose is required 2 to 4 weeks later.
Annual re-vaccination with a single 1 ml dose is recommended.
-
The vaccine should be stored at a temperature between 2 and 7 °C. Protect from light. Do not freeze.
-
Bronchicine™ is marketed in ten dose vials and packed as 5 x 10 dose vials.
DISCLAIMER: ALTHOUGH GREAT CARE HAS BEEN TAKEN IN THE COMPILATION OF THIS DOCUMENT, READERS ARE REQUESTED TO REFER TO THE PACKAGE INSERT FOR COMPLETE DETAILS BEFORE USING THE SPECIFIC PRODUCT.