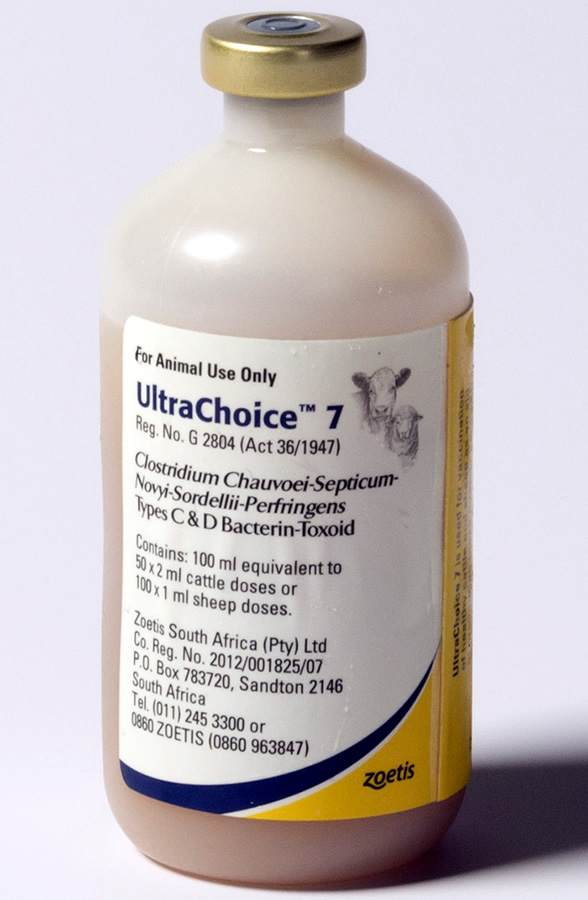
Do not vaccinate within 21 days before slaughter.
Keep out of reach of children, uninformed persons and animals.
As with most vaccines, anaphylaxis may occur after use. Initial antidote of adrenalin is recommended and should be followed with appropriate supportive therapy.
Transient local swelling at injection site may occur following vaccination.
This product has been shown to be efficacious in healthy animals. A protective immune response may not be elicited if animals are incubating an infectious disease, are malnourished or parasitized, are stressed due to shipment or environmental conditions, are otherwise immunocompromised, or the vaccine is not administered in accordance with label directions.
Milk production may be reduced in cows for up to six days following vaccination, thereafter milk production will return to normal.
Although this vaccine has been tested extensively under a large variety of conditions failure thereof may ensue as a result of a wide range of reasons. If this is suspected, seek veterinary advice and notify the registration holder.